
Is Fenbendazole Safe for Humans? Truth Behind the Antiparasitic Trend
Fenbendazole is not considered safe for human use according to the FDA and major medical bodies.
While some people are using it off-label, especially in cancer circles, there is no solid clinical evidence proving it’s safe or effective for humans.
There are serious risks, including liver damage. Let’s break down the facts, science, and expert opinions behind this growing trend.
What Is Fenbendazole and Its Original Veterinary Use?
Fenbendazole is a deworming medicine made for animals.
It’s used to treat worms like roundworms, hookworms, whipworms, and some tapeworms in dogs, cats, horses, and farm animals. It works by blocking how parasites grow inside the body.
It’s been used safely in animals for years, but it has never been tested or approved for use in people.
Is Fenbendazole FDA Approved for Humans?
No, Fenbendazole is not approved by the FDA or EMA for human use. There are no human dosage guidelines, no safety trials, and no licensed pharmaceutical versions for people.
Using veterinary drugs on humans is not only unsafe but also legally prohibited in most regions.
Health authorities warn against off-label use due to the lack of quality control, dosage clarity, and clinical evidence.
Related: Most Common Side Effects of Sildenafil: What You Need to Know
Why People Are Using Fenbendazole Off-Label
People are taking fenbendazole off-label due to claims that it may help treat cancer, despite lacking human studies.
This trend was largely popularized by Joe Tippens, a cancer patient who claimed remission after using the drug.
Interest is based on early lab studies that show potential anticancer effects, such as:
- Microtubule destabilization (affecting cancer cell structure)
- GLUT inhibition (blocking sugar supply to cancer cells)
- p53 gene activation (a known tumor suppressor)
While these mechanisms are promising in theory, they come from cell and animal studies, not human trials.
Also: A Complete Guide to ED Medications Online Available
Scientific Research: What Studies Actually Show
No proper human studies have proven that fenbendazole is safe or works in people. Most of the research is only in lab tests or on animals.
One woman, aged 67, had serious liver damage after taking fenbendazole for skin problems. Her liver levels went dangerously high, but they got better after she stopped using it.
Another 80-year-old woman, who had lung cancer, also had some liver issues while using it on her own.
These cases show real risks. Without proper studies on people, we don’t know how common or severe these problems could be.
Fenbendazole vs FDA-Approved Human Antiparasitic Medications
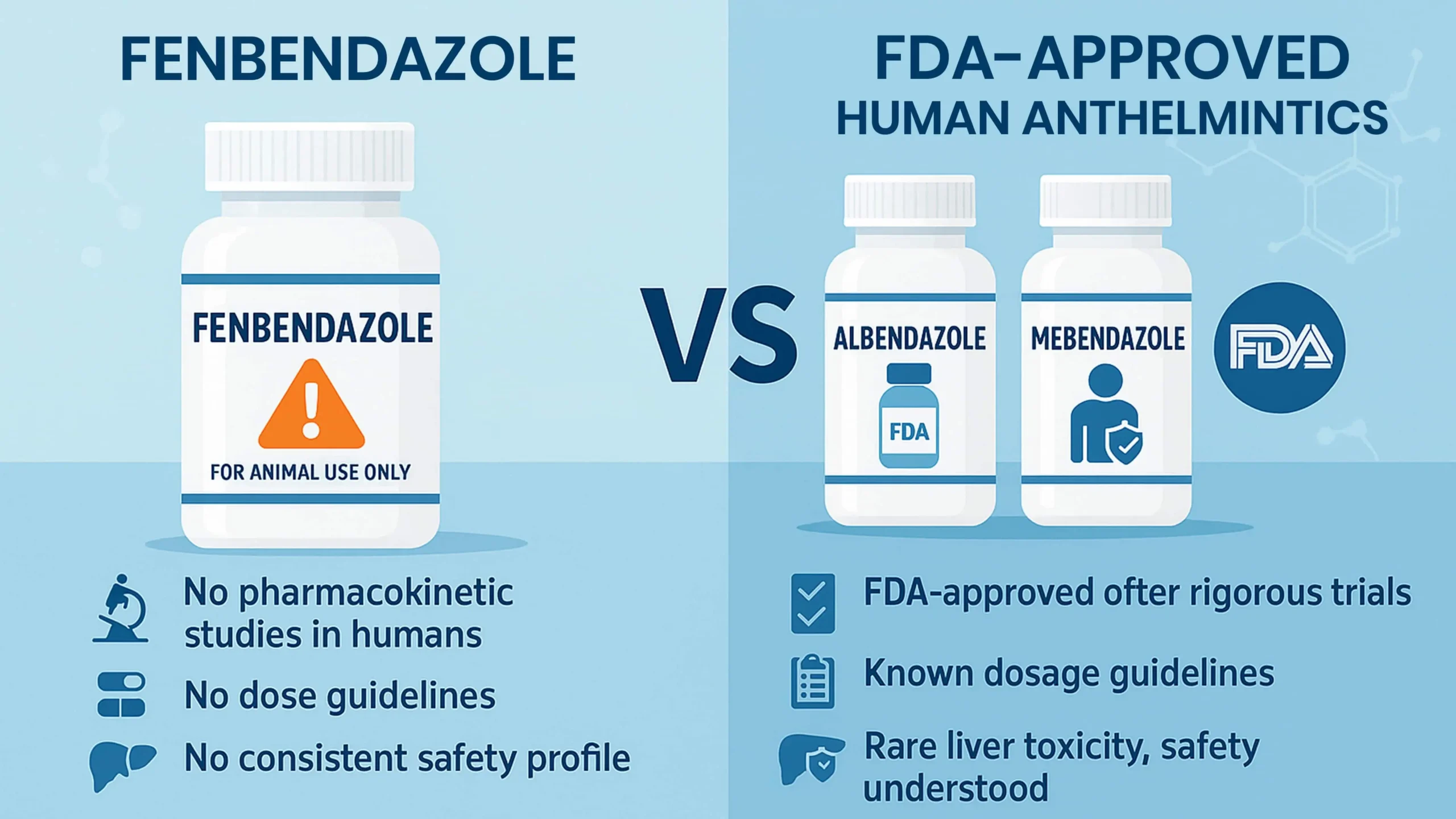
Albendazole and mebendazole are FDA-approved human anthelmintic drugs. They have undergone rigorous trials to determine safe dosages and side effects.
While both can cause rare liver toxicity, their safety is far better understood. Fenbendazole, by contrast, has:
- No pharmacokinetic studies in humans
- No dose guidelines
- No consistent safety profile
Despite chemical similarities, extrapolating data from albendazole to fenbendazole is risky and unsupported by evidence.
Medical Expert Warnings and Safety Concerns
Health agencies and medical experts strongly warn against the self-medication trend with fenbendazole.
- The FDA and EMA prohibit its use in humans due to the absence of safety trials.
- Oncologists and hepatologists have flagged fenbendazole-related liver injury as a serious concern.
- Experts stress that anecdotal stories do not replace clinical trials and that potential drug interactions (like with acetaminophen) could worsen outcomes.
Also: What Is Stronger: Sildenafil or Tadalafil? [Evidence-Based Comparison]
Legal and Ethical Considerations of Off-Label Use
Using veterinary drugs like fenbendazole on humans is not only risky but also illegal in many countries.
- Doctors cannot legally prescribe fenbendazole to humans.
- Dispensing or recommending it may violate professional and ethical medical standards.
- Ethically, off-label use should only be explored in regulated clinical trials, where risks and benefits are monitored.
Social media-driven misinformation, while emotionally compelling, can lead to harmful medical decisions.
Also check: Generic Ivermectin: Side Effects Guide
Final Thoughts
Fenbendazole is a trusted veterinary medication, but it is not safe or approved for humans.
The few human reports show risks, especially liver damage, and no clinical data support its use for cancer or other conditions.
Until human studies are conducted under ethical research settings, fenbendazole should not be used outside veterinary care.
People seeking alternative treatments should always consult licensed medical professionals.
FAQs
What is fenbendazole and what is it originally used for?
Fenbendazole is a veterinary deworming medication used to treat intestinal parasites such as roundworms, hookworms, whipworms, and some tapeworms in animals like dogs, cats, horses, and livestock. It works by disrupting parasite growth and reproduction.
Is fenbendazole approved for human use?
No. Fenbendazole is not approved by the FDA, EMA, or any major health authority for human use. There are no official human dosage guidelines, no safety studies, and no licensed pharmaceutical forms for people.
Why are some people taking fenbendazole off-label?
Some individuals use fenbendazole off-label due to anecdotal claims it may help treat cancer. This trend gained attention after cancer patient Joe Tippens shared his personal remission story. However, these claims are unsupported by human clinical trials.
Does scientific research support fenbendazole for cancer treatment?
No human studies confirm that fenbendazole is safe or effective for cancer. Existing research is limited to lab and animal studies showing potential anticancer mechanisms, but these results cannot be applied to people without proper clinical testing.
What are the potential risks and side effects of fenbendazole in humans?
The biggest known risk is liver damage, as seen in reported cases of people who self-medicated with fenbendazole. Other possible dangers include unknown drug interactions, incorrect dosing, and unpredictable side effects due to the lack of human safety data.
How does fenbendazole compare to FDA-approved human antiparasitic drugs?
FDA-approved human antiparasitic medications like albendazole and mebendazole have undergone rigorous safety testing, have established dosage guidelines, and are regulated for quality. Fenbendazole lacks such data, making its use in humans far riskier.
Have there been reports of liver damage from fenbendazole use in humans?
Yes. At least two documented cases show liver injury in people who took fenbendazole without medical supervision. In both cases, liver function improved after stopping the drug, highlighting its potential toxicity.
What do medical experts say about using fenbendazole in humans?
Medical experts, oncologists, and hepatologists strongly advise against fenbendazole use in humans. They warn that anecdotal reports are not reliable evidence and that unregulated use poses serious health risks.
Is it legal for doctors to prescribe fenbendazole to humans?
No. In most countries, prescribing or dispensing veterinary medicines like fenbendazole for human use is illegal. Such actions may violate medical ethics and regulatory laws unless part of an approved clinical trial.
What should people consider before trying fenbendazole for health conditions?
People should understand that fenbendazole is not approved for humans, has no proven benefits in people, and carries serious health risks. Anyone seeking alternative treatments should consult a qualified healthcare provider instead of self-medicating.

Aug 12, 2025
Generic and brand name medications work the same. They treat the same conditions and use the same main ingredients. The big difference is price generics…
VIEW DETAILSAug 12, 2025
Semaglutide helps people lose weight by lowering hunger and making them feel full for longer. It’s available as a weekly…
Aug 10, 2025
Ivermectin is a medicine used to treat several parasitic infections in humans, such as river blindness and intestinal worms. It…

